Non-alcoholic fatty liver disease (NAFLD) is a condition involving the accumulation of liver fat in excess of 5–10% of its weight after excluding excessive alcohol intake and any other causes of liver disease or steatosis.1 NAFLD has increased with the occurrence of obesity, diabetes, and metabolic syndrome, due to which an international consensus of experts proposed renaming it metabolic dysfunction-associated fatty liver disease (MAFLD).2,3 Liver steatosis and steatohepatitis, followed by advanced fibrosis, cirrhosis, and hepatocellular carcinoma, are pathological changes associated with MAFLD.4 One of the disease’s progressive phases is metabolic dysfunction-associated steatohepatitis (MASH), which is characterized by steatosis with ballooning and inflammation.5 Approximately a quarter of the world’s population has MAFLD, and a quarter of those patients have MASH.6 Despite its clinical significance, MASH has limited treatment options. Clinical drugs for MASH treatment are not yet available, necessitating the development of safe and effective MASH drugs and additional research.
Yinchenhao Decoction, which was introduced in Zhang Zhongjing’s “Treatise on Febrile Diseases”, is a traditional Chinese medicine prescribed for liver diseases.7 According to traditional Chinese medical beliefs, the main pathogenesis of MASH is dampness and heat stagnation. Si-Wei-Qing-Gan-Tang (SWQGT) is a folk prescription formulated from the Yinchenhao decoction and is used for clearing heat and dispelling dampness.8 The findings of our previous study showed that the effects of SWQGT are good for relieving MASH but are not satisfactory for reducing serum transaminases.8 Therefore, the improved formula, Bao-Gan-Xiao-Zhi-Wan (BGXZW), was studied. BGXZW consists of seven Chinese herbs: Artemisia capillaris Thunb, Hedyotis diffusa Willd, Gardenia jasminoides Ellis, Taxillus sutchuenensis (Lecomte) Danser, Salvia miltiorrhiza Bunge, Glycyrrhiza uralensis Fisch, and Rheum palmatum L (Supplementary Fig. 1A). The HPLC fingerprint of BGXZW (Supplementary Fig. 1B) shows that the main compounds were derived from Danshen, Yinchen, and Gancao.
In the present study, we established a rat model of MASH by feeding rats a methionine–choline-deficiency (MCD) diet. The methodology is described in detail in Supplementary File 1. As shown in Supplementary Table 1, the body weight of the rats in the model group was significantly lower (p<0.05) than those of the rats in the control group on Day 28. This loss of body weight was not reversed by BGXZW or SWQGT. The main reason for this observation was that the average food intake levels were lower for the model and drug rat groups than for the control rat group. In addition, the liver index was dramatically higher for the rats in the model group than for the rats in the control group (p<0.001), and BGXZW treatment reversed this elevation (p<0.05) (Supplementary Fig. 2B). The histological characteristics of the heart, spleen, lung, and kidney were observed. No significant difference was found in the morphological characteristics of the rats in each group (Supplementary Fig. 2F), indicating that BGXZW relieved the liver damage caused by the MCD diet.
Similar to SWQGT, administering BGXZW had certain effects on blood lipid levels (TC, TG, and LDL-c) (Fig. 1A–C). As ALT and AST are two important indicators for evaluating liver damage, we detected the serum and liver indicators of the MCD diet-fed rats (Fig. 1D–G). Results showed that the elevated ALT and AST levels caused by the MCD diet were remarkably reversed by BGXZW. In general, BGXZW was found to significantly increase blood fat levels, especially reduce the level of transaminase, better than SWQGT.
The morphology of the liver tissue in each rat group is shown in Figure 1H. Intuitive observations with the naked eye revealed that the rat livers in the control group were dark red, with normal capsules and clear edges, and they were elastic to the touch. In contrast, the rat livers in the model group were enlarged, soft, and grayish white, with tense capsules and blunt edges. Further, the rat livers in the BG-H and SW groups were superior to those in the model group in terms of shape, size, color, and touch. H&E staining revealed that the MCD diet induced macrovesicular steatosis and inflammation in the livers of the rats (Fig. 1I). Numerous fat vacuoles were observed in the liver tissue of the model group rats, and adjacent cells were crowded together, resulting in unclear cell boundaries. The nucleus was located on one side of the cell, and the structure of the liver cord was blurred. These conditions of liver fat accumulation caused by the MCD diet were attenuated by treatment with PPC, BGXZW, and SWQGT. However, liver fat percentage alone does not reflect the degree of liver inflammation, liver damage, or tissue fibrosis. Therefore, the NAFLD activity score (NAS) is generally used in clinical and preclinical studies. A total NAS score greater than 5 is indicative of MASH rather than simple fatty liver disease.9 Pathologists obtain the total NAS score by summing the scores of three parameters: steatosis (0–3 points), inflammation (0–3 points), and ballooning (0–2 points).9 Accordingly, we selected eight visual fields for scoring in each group; the scores and scoring specification are shown in Tables 1 and 2. The NAS score of the model group was 6.375±1.19, indicating that these rats suffered from typical MASH. In contrast, the rats in the PPC, BG, and SW groups had improved scores of less than 5. These results proved that BGXZW and SWQGT ameliorated the MCD-induced abnormal lipid accumulation and inflammatory injury in the livers of rats.
Table 1Effects of BGXZW and PPC on NAFLD Activity Score (NAS) in MCD diet-fed rats
| Groups | NAS Score
|
|---|
| Steatosis | Inflammation | Ballooning | Total |
|---|
| Ctrl group | 0 | 0 | 0 | 0 |
| Model group | 2.75±0.46### | 1.875±0.83### | 1.75±0.46### | 6.375±1.19### |
| PPC group | 1.625±0.52*** | 1.5±0.76 | 0.625±0.52*** | 3.75±1.04*** |
| BG-L group | 1.875±0.64** | 1.5 ±0.76 | 0.625±0.52*** | 4±1.30*** |
| BG-H group | 1.625±0.52*** | 1.375±0.74 | 0.5±0.53 | 3.5±1.07*** |
| SW group | 1.75±0.70** | 1.5±0.76 | 0.625±0.52*** | 3.875±0.99*** |
Table 2Scoring specification
| Classify | Histological features | Score |
|---|
| Healthy | 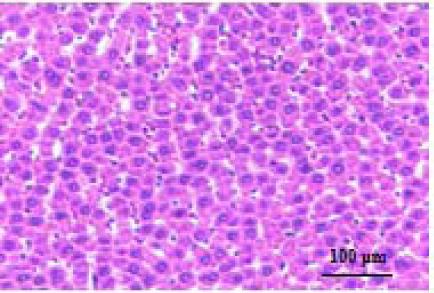 | Hepatocytes are nicely arranged and densely packed. |
| Steatosis | 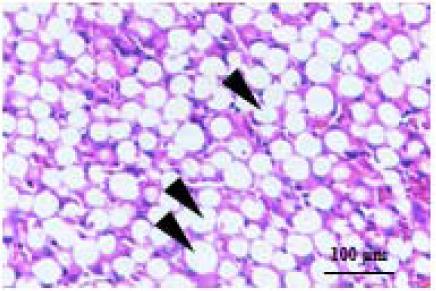 | Steatosis score represents the percentage of lipid droplets present in each field of view, 0 point, <5%; 1 point, <33%; 2 point, 34–66%; 3 point, >66 %. |
| Inflammation | 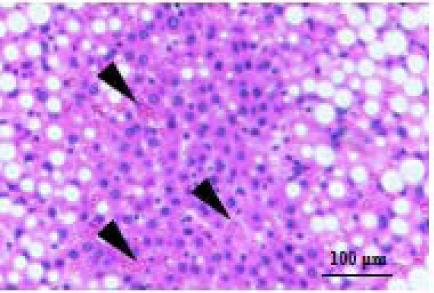 | Inflammation score represents the number of inflammatory cell clusters (1 cluster 1 foci), 0 point, 0 foci; 1 point,1–2 foci; 2 point, 3–4 foci; 3 point, >4 foci. |
| Ballooning | 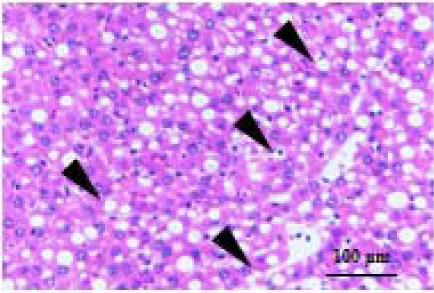 | Ballooning score is indicative of the number of hepatocytes that have altered cell structure due to excess lipid accumulation, 0 point, none; 1 point, few; 2 point, many. |
Nuclear factor-κB (NF-κB) is a transcription factor that plays a vital role in regulating the inflammatory response.10 To explore the underlying mechanism, we first examined the levels of NF-κB and downstream inflammatory factors. BGXZW treatment resulted in a large decrease in the levels of NF-κB, IL-1β, COX2, and iNOS (Fig. 2A, C-E). These experimental results confirmed that the MCD diet activated the NF-κB pathway and that BGXZW played an anti-inflammatory role in the liver by downregulating NF-κB and inhibiting the release of inflammatory factors.
Previous studies have shown that SWQGT inhibits the mechanistic target of rapamycin (mTOR) and activates autophagy in the livers of MCD diet-fed rats.8 Thus, we also examined autophagy-associated proteins and genes. ULK1 is a key protein for autophagy initiation, p62 is an autophagic substrate usually used to evaluate the level of autophagy flux, and LC3II is a marker of autophagosome formation; therefore, these three proteins are considered key proteins in the regulation of autophagy.11,12 As shown in Figure 2B, the rats in the BGXZW treatment group had decreased levels of p-mTOR and p62 proteins and enhanced levels of p-ULK1 and LC3II proteins. In addition, the rats in the BG-L and BG-H groups had reduced RNA levels of mTOR (p<0.01), compared to the rats in the model group (Fig. 2F). Furthermore, compared to the model group, the BG-H group exhibited increased RNA levels of LC3 (p<0.05), while the BG-L group showed no significant difference (Fig. 2G). In general, BGXZW inhibited mTOR-activated autophagy in the livers of the MCD diet-fed rats.
In sum, BGXZW effectively reduced serum ALT and AST levels, thus showing that the improved Chinese herbal formula is successful in alleviating MASH. The results of the preliminary molecular mechanism study confirmed that BGXZW attenuated MCD diet-induced MASH in rats by modulating the NF-κB signaling pathway and autophagy (Fig. 2H).
Supporting information
Supplementary Fig. 1
Components (A) and HPLC fingerprint (B) of BGXZW.
(TIF)
Supplementary Fig. 2
Effect of BGXZW and SWQGT on heart, spleen, lung, and kidney of rats in each group.
(A) Organ index; (B) HE stained sections, 100 ×. Results are expressed as the mean±SEM (n=8 rats in each group). #p<0.05 compared with the control group, ###p<0.001 compared with the control group, *p<0.05 compared with model group. Ctrl group, MCS diet and fed with pure water; group; Model group, MCD diet and fed with pure water; PPC group, MCD diet and fed with PPC (120 mg/kg/d); BG-L group, MCD diet and fed with low-dose BGXZW (1.67 g/kg/d); BG-H group, MCD diet and fed with high-dose BGXZW (5 g/kg/d); SW group, MCD diet and fed with SWQGT (3 g/kg/d). MCD, methionine choline deficiency; MCS, methionine and choline-sufficient; BGXZW, Bao-Gan-Xiao-Zhi-Wan; SWQGT, Si-Wei-Qing-Gan-Tang.
(TIF)
Supplementary File 1
Supplementary Materials.
(DOCX)
Supplementary Table 1
Effects of BGXZW and PPC on body weight in MCD diet-fed rats.
(DOCX)
Abbreviations
- ALT:
alanine aminotransferase
- AST:
aspartate transaminase
- BGXZW:
Bao-Gan-Xiao-Zhi-Wan
- H&E:
hematoxylin and eosin
- HPLC:
High Performance Liquid Chromatography
- LDL-c:
low-density lipoprotein cholesterol
- MCD:
methionine choline deficiency
- MCS:
methionine and choline-sufficient
- mTOR:
mechanistic target of rapamycin
- MAFLD:
metabolic dysfunction-associated fatty liver disease
- MASH:
metabolic dysfunction-associated steatohepatitis
- NAFLD:
non-alcoholic fatty liver disease
- NAS:
NAFLD activity score
- NF-κB:
nuclear factor-κB
- PCR:
Polymerase Chain Reaction
- SWQGT:
Si-Wei-Qing-Gan-Tang
- TC:
total cholesterol
- TG:
triglyceride
Declarations
Ethical statement
The animal experiments were approved by the Institutional Animal Care and Use Committee of the Shenzhen People’s Hospital (No. LL-KT-2021797) and all animals received humane care.
Data sharing statement
Supplementary Materials and Methods were added in Supplementary File 1. The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by the Basic and Applied Basic Research Foundation of Guangdong Province (2021A1515220185).
Conflict of interest
The authors have no conflict of interests related to this publication.
Authors’ contributions
Conceptualization, LZ, XW, LG, XZ and BZ; Data curation, LZ and JY; Formal analysis, WW and XZ; Investigation, LZ, XW, and WS; Methodology, LZ, XW, WS, JY and LG; Supervision, BZ; Writing-original draft, LZ; Writing-review & editing, LG and BZ.